Prevention of fibrotic liver disease progression: Urgent unmet need
Chronic fibrotic liver diseases can be caused by viral (e.g., hepatitis B virus [HBV], hepatitis C virus [HCV]) and metabolic (e.g., alcohol-related liver disease [ALD], non-alcoholic fatty liver disease [NAFLD], diabetes) etiologies. Cirrhosis is the terminal stage of the progressive liver fibrosis, which is highly prevalent (estimated to affect 1-2% of global population) and lethal by developing liver failure and liver cancer, the second leading cause of cancer mortality worldwide. Despite these clinically unequivocal high-risk conditions, prevention of the disease progression has been a challenging task as evidenced by the absence of chemoprevention therapy available in clinic.
The major hurdle is the lengthy process of disease progression toward cancer development, which typically takes two to three decades after initial exposure to the etiological agents. Even if candidate chemoprevention targets are identified in experimental animal models, their clinical validation is difficult or practically infeasible because of the time period far longer than the time frame of typical research study and clinical trial. In addition, it is logistically and ethically difficult to engage asymptomatic and cancer-free individuals for monitoring of cancer development for years. Indeed, all major chemoprevention clinical trials have failed to demonstrate their clinical benefit despite the requirement for large sample size and long observation periods.
Reverse-engineering chemoprevention discovery
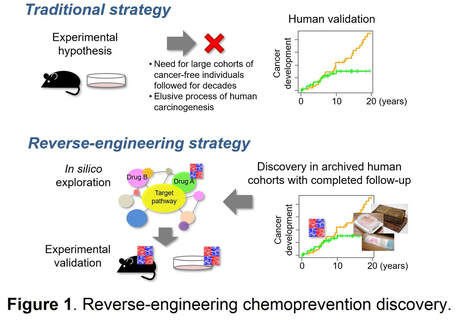
To overcome the challenge, we employ an innovative “reverse-engineering” approach, where clinically relevant targets are first discovered in human cohorts with already completed decades of clinical follow-up, and then experimentally evaluated for mechanisms and therapeutic strategies to minimize the risk of failure in the clinical testing phase (Figure 1). To enable this approach, we developed a genome-wide molecular profiling assay specialized for archived formalin-fixed tissue samples for the first time, and analyzed clinical specimens with more than two decades of completed clinical follow-up for development of lethal cirrhosis complication and cancer. Meta-analysis of the data from > 500 cirrhosis patients revealed approximately 30 regulatory gene modules that measure global molecular dysregulations and enable systematic identification targets/agents (e.g., epidermal growth factor signaling, lysophosphatidic acid pathway, pioglitazone) and cross-species comparison. Single-cell omics profiling combined with Digital Spatial Profiling (DSP) enables identification of more precise cellular mechanisms of disease progression and drug response. In collaboration with international multidisciplinary team (Precision Liver Cancer Prevention Consortium), we have successfully utilized this approach to identity several promising chemoprevention targets, some of which are now being tested in patients with cirrhosis.
Chronic fibrotic liver diseases can be caused by viral (e.g., hepatitis B virus [HBV], hepatitis C virus [HCV]) and metabolic (e.g., alcohol-related liver disease [ALD], non-alcoholic fatty liver disease [NAFLD], diabetes) etiologies. Cirrhosis is the terminal stage of the progressive liver fibrosis, which is highly prevalent (estimated to affect 1-2% of global population) and lethal by developing liver failure and liver cancer, the second leading cause of cancer mortality worldwide. Despite these clinically unequivocal high-risk conditions, prevention of the disease progression has been a challenging task as evidenced by the absence of chemoprevention therapy available in clinic.
The major hurdle is the lengthy process of disease progression toward cancer development, which typically takes two to three decades after initial exposure to the etiological agents. Even if candidate chemoprevention targets are identified in experimental animal models, their clinical validation is difficult or practically infeasible because of the time period far longer than the time frame of typical research study and clinical trial. In addition, it is logistically and ethically difficult to engage asymptomatic and cancer-free individuals for monitoring of cancer development for years. Indeed, all major chemoprevention clinical trials have failed to demonstrate their clinical benefit despite the requirement for large sample size and long observation periods.
Reverse-engineering chemoprevention discovery
To overcome the challenge, we employ an innovative “reverse-engineering” approach, where clinically relevant targets are first discovered in human cohorts with already completed decades of clinical follow-up, and then experimentally evaluated for mechanisms and therapeutic strategies to minimize the risk of failure in the clinical testing phase (Figure 1). To enable this approach, we developed a genome-wide molecular profiling assay specialized for archived formalin-fixed tissue samples for the first time, and analyzed clinical specimens with more than two decades of completed clinical follow-up for development of lethal cirrhosis complication and cancer. Meta-analysis of the data from > 500 cirrhosis patients revealed approximately 30 regulatory gene modules that measure global molecular dysregulations and enable systematic identification targets/agents (e.g., epidermal growth factor signaling, lysophosphatidic acid pathway, pioglitazone) and cross-species comparison. Single-cell omics profiling combined with Digital Spatial Profiling (DSP) enables identification of more precise cellular mechanisms of disease progression and drug response. In collaboration with international multidisciplinary team (Precision Liver Cancer Prevention Consortium), we have successfully utilized this approach to identity several promising chemoprevention targets, some of which are now being tested in patients with cirrhosis.
Experimental validation in patient-derived materials
To complete the process of reverse-engineering chemoprevention discovery, identified candidate targets, biomarkers, and/or chemoprevention/anti-fibrotic agents are validated in patient-derived materials. We regularly access fresh clinical liver tissues affected with various chronic liver diseases such as viral hepatitis and NAFLD from patients with diverse racial/ethnic background. Functional assessment of the targets/agents is performed in various experimental models such as primary cells, PLS-inducible cells, and organotypic ex vivo tissue culture in combination with various omics technologies such as single-cell sequencing and digital spatial profiling.
References
1. Rasha F, Paul S, Simon TG, Hoshida Y. Hepatocellular carcinoma chemoprevention with generic agents. Semin Liver Dis. 2022.
2. Crouchet E, Bandiera S, Fujiwara N, et al. A human liver cell-based system modeling a clinical prognostic liver signature for therapeutic discovery. Nat Commun. 2021 Sep 17;12(1):5525.
3. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526-49.
4. Athuluri-Divakar SK, Hoshida Y. Generic chemoprevention of hepatocellular carcinoma. Ann N Y Acad Sci. 2019;1440(1):23-35.
5. Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30(6):879-90.
6. Crouchet E, Li S, Sojoodi M, et al. Hepatocellular carcinoma chemoprevention by targeting the angiotensin-converting enzyme and EGFR transactivation. JCI Insight. 2022 Jul 8;7(13):e159254..
7. Kim MH, Kim MY, Salloum S, et al. Atorvastatin favorably modulates a clinical hepatocellular carcinoma risk gene signature. Hepatol Commun. 2022.
8. Li S, Ghoshal S, Sojoodi M, Arora G, Masia R, Erstad DJ et al. Pioglitazone Reduces Hepatocellular Carcinoma Development in Two Rodent Models of Cirrhosis. J Gastrointest Surg. 2019;23(1):101-11.
9. Bansal R, Nakagawa S, Yazdani S, van Baarlen J, Venkatesh A, Koh AP et al. Integrin alpha 11 in the regulation of the myofibroblast phenotype: implications for fibrotic diseases. Exp Mol Med. 2017;49(11):e396.
Past and current grants
NIH/NCI R01 CA282178, Hoshida, Singal (MPI)
NIH/NCI U01 CA288375, Hoshida, Chung, Moylan, Diehl (MPI)
NIH/NCI R01 CA233794, Hoshida (PI)
NIH/NCI R01 CA255621, Chung, Hoshida (MPI)
CPRIT, RR180016, Hoshida (PI)
European Research Council, 671231 HEPCIR, Baumert (PI)
Irma T. Hirschl/Monique Weill-Caulier Scholar Award, Hoshida (PI)
U.S. Department of Defense, W81XWH-16-1-0363, Hoshida (PI)
Clinical trials
NCT02273362, Tanabe, Limburg (co-PIs)
NCT04172779, Hoshida, Singal (co-PIs)
NCT05028829, Chung, Hoshida (co-PIs)