Regular HCC screening is a challenging task
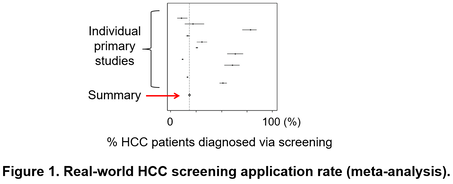
Hepatocellular carcinoma (HCC) develops almost exclusively from chronically diseased livers due to viral and metabolic etiologies at extremely high frequency, 1-7% annually especially when cirrhosis is present. Therefore, it is rational to regularly monitor the patients for development of HCC and other lethal complications for their early detection and intervention to improve survival. Practice guidelines thus recommend regular HCC screening (or interchangeably, surveillance) with biannual ultrasound and serum tumor marker, alpha-fetoprotein (AFP). However, this “one-size-fits-all” approach is too much demand for currently available medical resources as evidenced by the low adherence rate (only 18%) in real-world clinical setting (Figure 1).
Hepatocellular carcinoma (HCC) develops almost exclusively from chronically diseased livers due to viral and metabolic etiologies at extremely high frequency, 1-7% annually especially when cirrhosis is present. Therefore, it is rational to regularly monitor the patients for development of HCC and other lethal complications for their early detection and intervention to improve survival. Practice guidelines thus recommend regular HCC screening (or interchangeably, surveillance) with biannual ultrasound and serum tumor marker, alpha-fetoprotein (AFP). However, this “one-size-fits-all” approach is too much demand for currently available medical resources as evidenced by the low adherence rate (only 18%) in real-world clinical setting (Figure 1).
Risk-stratified HCC screening mitigates the burden and is cost-effective
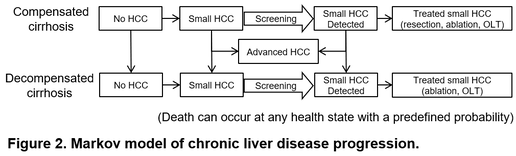
Individual-risk-based personalized HCC screening is theoretically a rational approach to address the challenge by optimizing allocation of the limited medical resources to those who most need it. To quantitatively evaluate benefit of this personalized “precision medicine” approach, we developed Markov-chain-based models to simulate natural history of liver disease progression starting from diagnosis of compensated cirrhosis to death, and thoroughly compared various risk-stratified strategies with the guideline-recommended “one-size-fits-all” strategy (Figure 2).
Individual-risk-based personalized HCC screening is theoretically a rational approach to address the challenge by optimizing allocation of the limited medical resources to those who most need it. To quantitatively evaluate benefit of this personalized “precision medicine” approach, we developed Markov-chain-based models to simulate natural history of liver disease progression starting from diagnosis of compensated cirrhosis to death, and thoroughly compared various risk-stratified strategies with the guideline-recommended “one-size-fits-all” strategy (Figure 2).
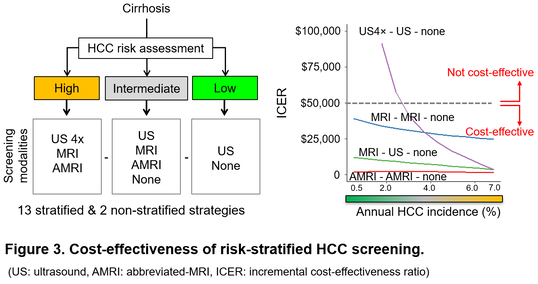
Of note, several risk-stratified strategies extend net patient survival with minimal increase of whole medical care costs, especially when new imaging modality such as abbreviated-MRI (AMRI) tailored for HCC screening, is utilized (Figure 3). For example, the measure of cost-effectiveness, incremental cost-effectiveness ratio (ICER), is only $2,100 to gain one additional quality-adjusted life year when AMRI is applied to patients with higher HCC risk compared to the rest. Such computational modeling guides development of risk-predictive biomarkers/algorithms and screening modalities by providing benchmark costs and performance to aim to make each personalized HCC screening strategy cost-effective.
References
1. Lee YT, Fujiwara N, Yang JD, Hoshida Y. Risk stratification and early detection biomarkers for precision hepatocellular carcinoma screening. Hepatology. 2022.
2. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526-49.
3. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-50.
4. Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-7.
5. Goossens N, Bian CB, Hoshida Y. Tailored algorithms for hepatocellular carcinoma surveillance: Is one-size-fits-all strategy outdated? Curr Hepatol Rep. 2017;16(1):64-71.
6. Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017;8(6):e101.
7. Khatri G, Pedrosa I, Ananthakrishnan L, de Leon AD, Fetzer DT, Leyendecker J et al. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. Journal of magnetic resonance imaging : JMRI. 2019.
8. Besa C, Lewis S, Pandharipande PV, Chhatwal J, Kamath A, Cooper N et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY). 2017;42(1):179-90.
9. Lee JY, Huo EJ, Weinstein S, Santos C, Monto A, Corvera CU et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol (NY). 2018;43(7):1627-33.
Past and current grants
NIH/NCI U01 CA283935, Singal, Hoshida (MPI)
CPRIT, RR180016, Hoshida (PI)
CPRIT, RP200554, Singal, Hoshida (MPI)
NIH/NCI R01 CA212008, Singal (PI)
NIH/NCI U01 CA230694, Singal (PI)
Irma T. Hirschl/Monique Weill-Caulier Scholar Award, Hoshida (PI)
NIH/NIDDK R01 DK099558, Hoshida (PI)
1. Lee YT, Fujiwara N, Yang JD, Hoshida Y. Risk stratification and early detection biomarkers for precision hepatocellular carcinoma screening. Hepatology. 2022.
2. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526-49.
3. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-50.
4. Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-7.
5. Goossens N, Bian CB, Hoshida Y. Tailored algorithms for hepatocellular carcinoma surveillance: Is one-size-fits-all strategy outdated? Curr Hepatol Rep. 2017;16(1):64-71.
6. Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017;8(6):e101.
7. Khatri G, Pedrosa I, Ananthakrishnan L, de Leon AD, Fetzer DT, Leyendecker J et al. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. Journal of magnetic resonance imaging : JMRI. 2019.
8. Besa C, Lewis S, Pandharipande PV, Chhatwal J, Kamath A, Cooper N et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY). 2017;42(1):179-90.
9. Lee JY, Huo EJ, Weinstein S, Santos C, Monto A, Corvera CU et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol (NY). 2018;43(7):1627-33.
Past and current grants
NIH/NCI U01 CA283935, Singal, Hoshida (MPI)
CPRIT, RR180016, Hoshida (PI)
CPRIT, RP200554, Singal, Hoshida (MPI)
NIH/NCI R01 CA212008, Singal (PI)
NIH/NCI U01 CA230694, Singal (PI)
Irma T. Hirschl/Monique Weill-Caulier Scholar Award, Hoshida (PI)
NIH/NIDDK R01 DK099558, Hoshida (PI)